Cell-cycle dependent cell-type choice
How can a group of undifferentiated cells break symmetry and become different cell types?
If the cells are at different phases of the cell cycle, a signal can tell them to look at what phase they happen to be in and this will determine their initial fate.
In Dictyostelium, initial cell type choice is correlated with the cell-cycle phase that the cell happens to be in at the time of starvation. For each pair of sister cells which happen to be in S or early G2 phase (Dictyostelium does not have an appreciable G1 phase) when they starve, one sister differentiates into a prestalk cell while the other sister becomes a null cell (a cell that does not express either a characteristic prestalk or a prespore antigen). Cells starved in late G2 or M phase similarly differentiate into either a prespore or a null cell.
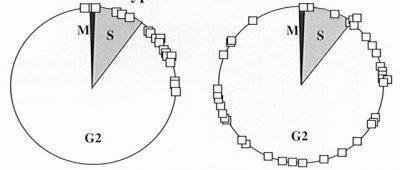
Origin of prestalk cells in a population of wild-type (left) and rtoA¯ cells (right). A population of cells, where the cells were at random phases of the cell cycle, was starved and allowed to differentiate into prestalk and prespore cells. The squares mark where the cells that became prestalk happened to be in the cell cycle at the time of starvation.
We have isolated a mutant, ratioA (rtoA¯), with a defect in this mechanism, and this results in an abnormally high percentage of prestalk cells. The cell cycle is normal in rtoA¯ cells. In the wild type, prestalk cells differentiate from those cells in S or early G2 phase at starvation and prespore cells from cells in late G2 or M phase at starvation. In rtoA¯ mutants, both prestalk and prespore cells originate randomly from cells in any phase of the cell cycle at starvation. We found that rtoA¯ cells have a defect in the fusion of endocytic vesicles. They also have a decreased exocytosis rate, a decreased pH of endocytic/exocytic vesicles, and an increased average cytosolic pH. Our data indicate that the serine-rich domain of RtoA can mediate membrane fusion and that RtoA can increase the rate of vesicle fusion during processing of endoctyic vesicles. We hypothesize that RtoA modulates initial cell type choice by linking vegetative cell physiology to the cell cycle.
This ‘musical chairs’ model of initial cell type differentiation generated a good bit of opposition, as it ran counter to a hypothesis that cells remained undifferentiated as they formed an aggregate, and then a morphogen gradient in the aggregate would tell cells what to differentiate into. Over the years, a variety of experiments from other groups has disproved the morphogen gradient model and supported the musical chairs model for initial cell type choice.
Key Papers
Gomer, R.H., and Firtel, R.A. Cell-autonomous determination of cell-type choice in Dictyostelium development by cell-cycle phase. Science 237, 758-762 (1987).
Clay, J., Ammann, R., and Gomer, R.H. Initial cell-type choice in a simple eukaryote: Cell-autonomous or morphogen-gradient dependent? Dev. Biol. 172, 665-674 (1995).
Gomer, R.H. and Ammann, R. A cell-cycle phase-associated cell-type choice mechanism monitors the cell cycle rather than using an independent timer. Dev. Biol. 174, 82-91 (1996).
Wood SA, Ammann RR, Brock DA, Li L, Spann T, Gomer RH. RtoA links initial cell type choice to the cell cycle in Dictyostelium. Development 122:3677-85 (1996)
Brazill DT, Caprette DR, Myler HA, Hatton RD, Ammann RR, Lindsey DF, Brock DA, Gomer RH. A protein containing a serine-rich domain with vesicle fusing properties mediates cell cycle-dependent cytosolic pH regulation. J Biol Chem 275:19231-40 (2000).
Azhar, M., Kennady, P.K., Pande, G., Espiritu, M., Holloman, W., Brazill, D., Gomer, R.H., and Nanjundiah, V. The phase of the cell cycle at starvation influences the level of cellular Ca2+ and, thereby post-aggregative cell fate in Dictyostelium discoideum. Int. J. Dev. Bio. 44, 405-414 (2001).
Brazill, D.T., Meyer, L.R., Hatton, R.D., Brock, D.A., and Gomer, R.H. ABC transporters required for endocytosis and endosomal pH regulation in Dictyostelium. J. Cell Sci. 114, 3923-3932 (2001).
